WBCIL’s R&D Innovation: Crafting Superior Iron APIs
WBCIL’s R&D innovation team is at the forefront of iron API innovation, leveraging cutting-edge technology and scientific expertise to deliver products that meet the highest standards of quality and efficacy.
Iron is a vital micronutrient essential for numerous physiological functions, including oxygen transport, energy production, and immune system development. Iron deficiency anaemia, a prevalent global health issue, underscores the criticality of accessible and effective iron supplementation [1,2]. WBCIL’s R&D innovation team is committed to addressing this challenge by developing superior iron APIs that enhance absorption, bioavailability, and overall patient outcomes.
A Focus on WBCIL’s R&D Innovation and Quality
WBCIL’s R&D approach is characterized by a relentless pursuit of innovation and quality. The team’s dedication to scientific excellence is evident in its rigorous research and development processes. Here are some key aspects of WBCIL’s R&D strategy:
- Advanced Research Techniques: WBCIL employs state-of-the-art research techniques, including computational modelling, in vitro testing, and in vivo studies, to gain a deep understanding of iron absorption mechanisms and to identify potential improvements.
- Novel Formulations: The R&D team explores novel formulations and delivery systems to enhance iron bioavailability and reduce gastrointestinal side effects [3]. This includes the development of controlled-release formulations, iron chelates, and iron-amino acid complexes.
- Quality Assurance: WBCIL maintains stringent iron API quality control standards throughout the entire manufacturing process [4,5]. This ensures that the iron APIs meet the highest regulatory requirements and are free from impurities.

Understanding Iron APIs: A Brief Overview
An iron API (Active Pharmaceutical Ingredient) is a purified iron supplement ingredient that is used as iron salts in pharmaceuticals. The choice of iron API can significantly impact the absorption, bioavailability, and tolerability of iron supplements. WBCIL’s R&D team has focused on developing iron APIs with superior properties, such as:
- High Bioavailability: The chosen iron API should be readily absorbed by the body to ensure effective iron supplementation and use of Iron-based APIs for anaemia treatment [6].
- Reduced Side Effects: The iron API should be formulated to minimize gastrointestinal side effects, such as constipation and nausea [7].
- Stability: The iron API should be stable under various storage conditions to maintain its potency and efficacy.
By carefully selecting and optimizing iron APIs, WBCIL is able to create iron supplements that are both effective and well-tolerated by patients.
The Role of Iron in Human Health
Iron is a vital trace mineral that plays a crucial role in numerous physiological functions. It is essential for the production of haemoglobin, a protein that carries oxygen to cells throughout the body [8,9]. Iron deficiency anaemia, a condition characterized by a low red blood cell count due to insufficient iron, can lead to a variety of symptoms, including fatigue, weakness, and shortness of breath.
The Challenges of Iron Supplementation
While iron supplements are widely available, they can be challenging to use effectively. Traditional iron supplements often have low absorption rates and can cause gastrointestinal side effects, such as constipation and nausea [10]. These factors can limit patient adherence to iron supplementation regimens.
WBCIL’s Innovative Approach
WBCIL’s R&D innovation team has focused on addressing the limitations of traditional iron supplements through the advanced iron API development. These innovative iron drug formulations are designed to enhance iron absorption, reduce side effects, and improve overall patient outcomes [11]. Uses of iron APIs in pharmaceuticals is wide to treat iron deficiency anemia and other iron-related conditions.
Our high-quality iron APIs are ideal for use in a variety of pharmaceutical products, including iron supplements, iron drug formulations, multivitamins, and specialized iron-fortified foods [12]. By partnering with WBCIL, pharmaceutical companies can access superior pharmaceutical iron APIs to create effective and well-tolerated products that meet the needs of their patients.
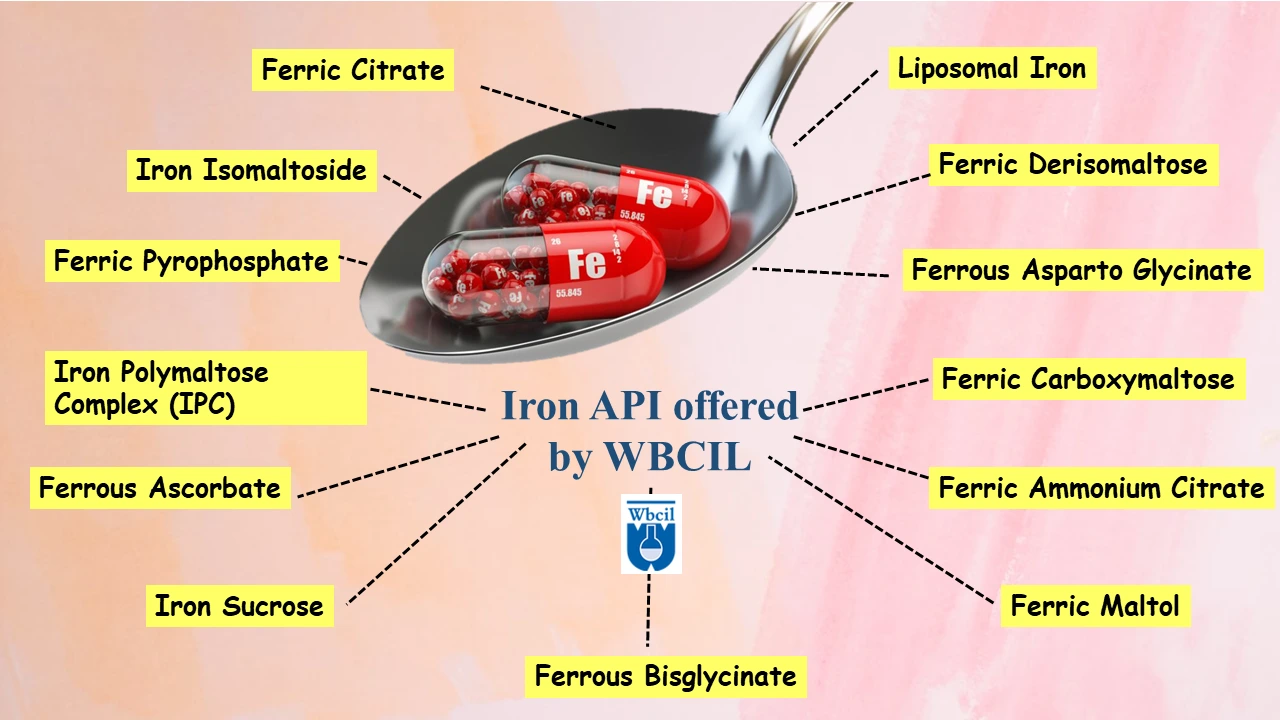
Iron API offered by WBCIL
- Liposomal Iron API
- ferric maltol API
- Ferric Citrate API
- Ferric Carboxymaltose API
- Ferrous Bisglycinate API
- Ferrous Ascorbate API
- Iron III Hydroxide Polymaltose Complex API
- Iron Isomaltoside API
- Ferric Orthophosphate API
- Ferric Derisomaltose API
- Ferrous Asparto Glycinate API
- Ferric Pyrophosphate Citrate API
- Ferric Ammonium Citrate API
- Iron Sucrose API
- Ferric Pyrophosphate API
- Sucroferric Oxyhydroxide API
Driving Innovation Through Collaboration
WBCIL recognizes the importance of collaboration in driving innovation. The R&D team actively engages with academic institutions, research organizations, and industry partners to share knowledge, explore new ideas, and accelerate product development. This collaborative approach fosters a dynamic and innovative research environment.
The Impact of WBCIL’s Innovation
WBCIL’s R&D efforts have resulted in the development of superior iron APIs that offer significant benefits to patients and healthcare providers. These innovations include:
- Enhanced Absorption: WBCIL’s iron APIs are formulated to maximize absorption in the gastrointestinal tract, ensuring that patients receive the full benefits of iron supplementation [13].
- Reduced Side Effects: WBCIL’s R&D team has developed formulations that minimize gastrointestinal side effects, such as constipation and nausea, often associated with traditional iron supplements [14].
- Improved Patient Outcomes: WBCIL’s innovative iron APIs contribute to improved patient outcomes by addressing iron deficiency anaemia effectively and efficiently [15].
Future Directions for Iron API Research
WBCIL’s R&D team continues to explore new avenues for iron API innovation. Future research may focus on:
- Developing iron APIs that are specifically tailored to different patient populations, such as children, pregnant women, and the elderly.
- Investigating the potential of iron APIs in combination with other nutrients or medications to improve overall health outcomes.
- Exploring novel delivery systems for iron APIs, such as nanoparticles or liposomes, to enhance absorption and reduce side effects.
Conclusion
WBCIL’s R&D innovation team is at the forefront of iron API innovation, dedicated to developing products that meet the highest standards of quality, efficacy, and safety. Through its commitment to scientific excellence, collaboration, and continuous improvement, WBCIL is making a significant contribution to global health by addressing the critical issue of iron deficiency anaemia.
1. https://www.wbcil.com/
2. https://www.ptinews.com/press-release/wbcil–a-legacy-of-innovation-and-excellence-in-pharmaceutical-manufacturing/1684907
3. https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/iron-salt
4. https://www.sciencedirect.com/topics/nursing-and-health-professions/iron-salt
5. Wikipedia contributors. Iron supplement. Wikipedia, The Free Encyclopedia. June 21, 2024, 21:07 UTC. Available at: https://en.wikipedia.org/w/index.php?title=Iron_supplement&oldid=1230289032. Accessed August 5, 2024.
6. https://livogen.in/which-iron-salt-is-better
7. Singhal S R, Kadian V, Singh S, Ghalaut V S, Comparison of Various Oral Iron Salts in the Treatment of Iron Deficiency Anemia in Pregnancy. Indian J Obstet Gynecol Res 2015;2(3):155-158. https://www.ijogr.org/journal-article-file/769
8. https://elsevier.health/en-US/preview/iron-salts
9. https://www.acgih.org/iron-salts-soluble/
10. Nguyen M, Tadi P. Iron Supplementation. [Updated 2023 Jul 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557376/
11. https://www.osha.gov/chemicaldata/499
12. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-. Iron Salts. [Updated 2024 Jan 15]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK575923/
13. Stroek, W. and Hoareau, L. and Albrecht, From the bottle: simple iron salts for the efficient synthesis of pyrrolidines via catalytic C–H bond amination, Catal. Sci. Technol., 2023,13, 958-962. https://pubs.rsc.org/en/content/articlelanding/2023/cy/d2cy02065c
14. https://www.cdc.gov/niosh/npg/npgd0346.html
15. https://medicalguidelines.msf.org/en/viewport/EssDr/english/ferrous-salts-16683659.html
16. Tarczykowska A, Engström N, Dobermann D, Powell J, Scheers N. Differential Effects of Iron Chelates vs. Iron Salts on Induction of Pro-Oncogenic Amphiregulin and Pro-Inflammatory COX-2 in Human Intestinal Adenocarcinoma Cell Lines. International Journal of Molecular Sciences. 2023; 24(6):5507. https://doi.org/10.3390/ijms24065507
17. https://www.britannica.com/science/iron-salt
