Ferric Carboxymaltose: The Future of Iron Deficiency Treatment
Ever felt like you’re running on empty, no matter how much you sleep? Feeling tired, weak, or short of breath? It could be your iron levels playing tricks on you. You might be suffering from a hidden energy crisis – iron deficiency.
Imagine a secret weapon that could supercharge your body, giving you a burst of energy and making you feel invincible. Iron, the unsung hero of our bodies, is essential for carrying oxygen to our cells. But sometimes, traditional oral iron supplements just don’t cut it, especially when you’ve had surgery, are battling a chronic illness like kidney disease or heart failure, or simply struggle to absorb iron from pills. That’s where ferric carboxymaltose (FCM) comes in [1].
This powerful iron infusion can bypass your digestive system and deliver iron directly to your bloodstream. Whether you need a quick boost after surgery or long-term support for a chronic condition, FCM could be your secret to feeling revitalized. Let’s dive into the world of FCM and unlock the power of this iron superhero.
What is Ferric Carboxymaltose?
Ferric Carboxymaltose (FCM) is a revolutionary intravenous iron therapy that redefines the standard of care for iron deficiency. It’s remarkably safe, with a low risk of allergic reactions, including anaphylaxis, setting it apart from other injectable iron formulations. This safety profile, combined with its efficacy, makes FCM a preferred choice for healthcare providers and patients alike [2,3].
It is a complex structure of iron (III) and a carbohydrate carrier, providing a highly stable and biocompatible solution that facilitates the efficient delivery of iron to the body [4]. It has CAS No.- 9007-72-1, with molecular Formula C24H44FeO25– and molecular Weight 788.4 g/m. Unlike oral iron supplements, which require absorption in the gastrointestinal tract, FCM, a parental iron treatment can be administered directly into the bloodstream, offering rapid restoration of iron levels, especially in patients with malabsorption issues or those who cannot tolerate oral treatments [5].
Advantages of Ferric Carboxymaltose Over Traditional Iron Supplements
Ferric carboxymaltose (FCM) in anemia treatment offers several advantages over traditional oral iron supplements, making it a preferred choice for many patients:
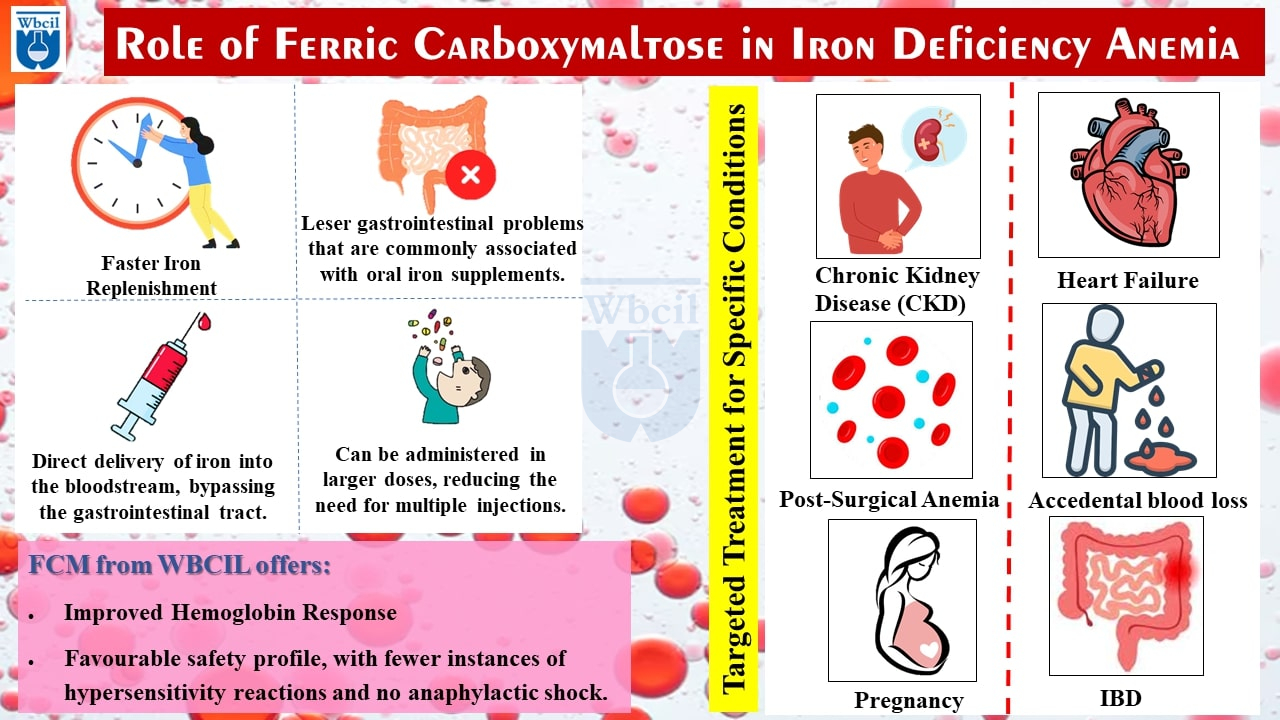
- Rapid and Efficient Iron Delivery: FCM is administered directly into the bloodstream, bypassing the digestive system [6]. This ensures rapid and efficient iron replenishment, especially in patients with malabsorption issues or those who cannot tolerate oral treatments.
- Superior Safety Profile: FCM has a low risk of allergic reactions, including anaphylaxis, setting it apart from other injectable iron formulations. This makes it a safer option for many patients [7,8].
- Improved Patient Compliance: The rapid onset of action and reduced side effects associated with FCM can lead to improved patient compliance with iron therapy treatment [9].
- Convenience and Flexibility: FCM’s flexibility is another key advantage. It can be administered in high doses in a single infusion, reducing the need for multiple sessions, and offering patients a more convenient treatment experience compared to other intravenous iron formulations [10].
- Targeted Treatment for Specific Conditions: FCM is particularly beneficial for:
- Chronic Kidney Disease (CKD): It can improve iron deficiency and reduce the need for erythropoietin-stimulating agents (ESAs).
- Heart Failure: FCM can address iron deficiency and exercise capacity in heart failure patients.
- Post-Surgical Blood loss: It can accelerate recovery and reduce the need for blood transfusions.
- Accidental blood loss: Replenishes iron stores promptly [11].
- Inflammatory Bowel Disease (IBD): FCM can bypass the gastrointestinal tract and directly deliver iron to the bloodstream, especially in patients with malabsorption issues.
- Pregnant Women: FCM can help address iron deficiency anemia, a common condition during pregnancy that can impact both maternal and fetal health [12].

The Role of WBCIL in Ferric Carboxymaltose Development
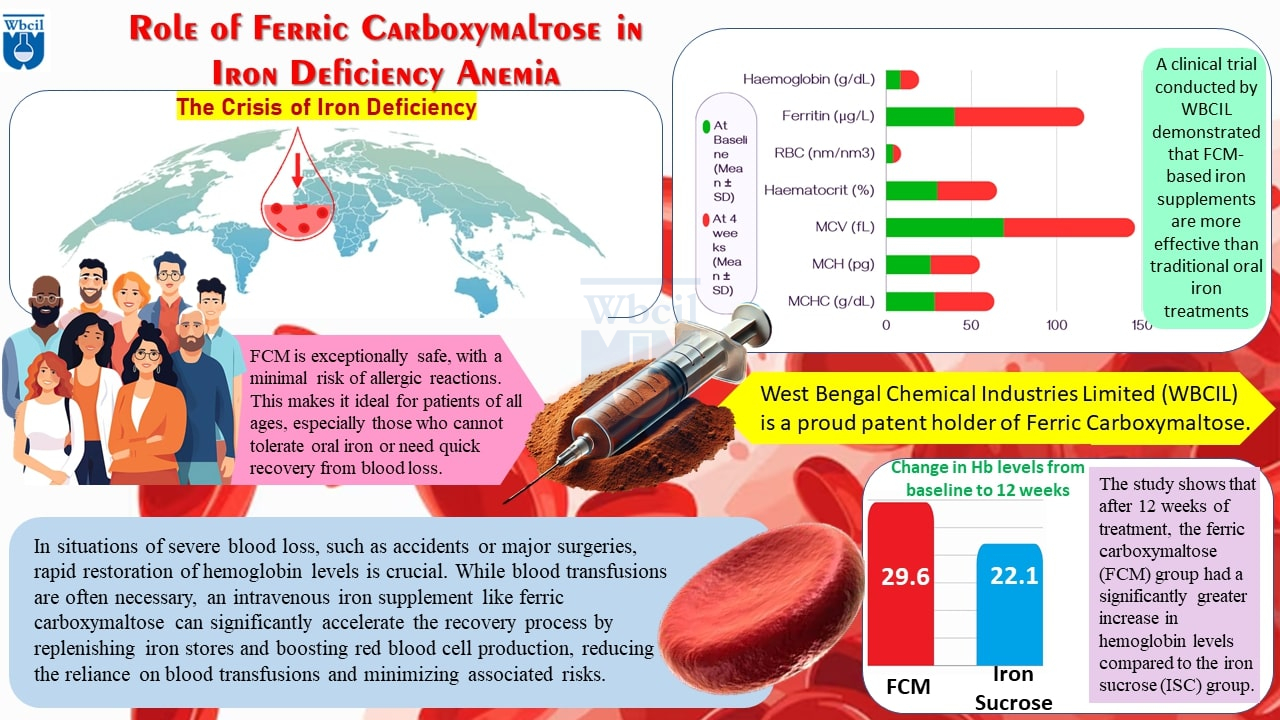
West Bengal Chemical Industries Limited (WBCIL) has been at the forefront of the development, manufacturing of ferric carboxymaltose. As a leading API manufacturer, WBCIL prioritizes strict CGMP and ISO quality control measures throughout the production process. The prolonged experience from 1962 ensures consistent potency, purity, and safety in every dose of WBCIL’s ferric carboxymaltose. As one of India’s leading manufacturers of Active Pharmaceutical Ingredients (APIs), WBCIL has leveraged its extensive research and development (R&D) capabilities to produce a high-quality, cost-effective version of ferric carboxymaltose. West Bengal Chemical Industries Limited (WBCIL) is a proud patent holder of Ferric Carboxymaltose [13].
WBCIL’s commitment to innovation has led to several groundbreaking advancements in the field of iron deficiency treatment, with their proprietary manufacturing processes ensuring the highest purity standards and therapeutic efficacy. The company’s dedication to quality has earned it recognition as a key player in the global market for iron supplementation.
WBCIL’s Research and Publications on Ferric Carboxymaltose
In recent years, WBCIL iron API and WBCIL API products has made significant contributions to the scientific community through the publication of research that delves into the effectiveness, safety profile, and comparative advantages of ferric carboxymaltose. The company’s research team has published two notable journal articles that explore various aspects of FCM, from its pharmacokinetics to its clinical application in different populations.
- A clinical trial conducted by WBCIL investigated the potential of Ferric Carboxymaltose (FCM) in the treatment of Iron Deficiency Anemia (IDA). The study involved 150 adult participants with IDA, who were randomly assigned to receive either an FCM-based iron supplement or a standard oral iron treatment. Over the course of 12 weeks, the trial measured changes in serum ferritin, hemoglobin levels, and gastrointestinal tolerance. Findings showed that FCM-based formulations were more effective in improving iron absorption and restoring iron levels compared to traditional iron supplements. This study reinforces WBCIL’s commitment to exploring innovative approaches to IDA management [14].
Ferric Carboxymaltose (FCM) and Iron Sucrose are intravenous iron formulations for iron deficiency anemia. A recent clinical trial by WBCIL compared their efficacy. FCM, often a single high-dose infusion, showed superior hemoglobin increase over Iron Sucrose, especially in pregnant women. While both have potential side effects, FCM is generally well-tolerated. The choice between them should be made by a healthcare provider considering individual patient needs [15].
These articles underscore the growing importance of ferric carboxymaltose inj uses in addressing the global burden of iron deficiency anemia. The research also emphasizes the therapeutic benefits of FCM in comparison to other non-inferiority intravenous iron formulations, such as iron sucrose, ferric derisomaltose and iron isomaltoside. Through these publications, WBCIL has helped educate both the medical community and the public about the advantages of using FCM as a treatment option for iron deficiency [16,17].
WBCIL’s research highlights the following key aspects of FCM
- Improved Hemoglobin Response: Several studies show that FCM leads to a faster and more substantial increase in hemoglobin levels compared to other iron supplements, which is crucial in treating severe cases of iron deficiency anemia [18,19].
- Safety Profile: Clinical trials have consistently shown that FCM has a favourable safety profile, with fewer instances of hypersensitivity reactions and other adverse events compared to older intravenous administration [20,21,22].
- Economic Impact: The cost-effectiveness of FCM is another major consideration. By reducing the need for multiple hospital visits and oral iron treatments, FCM presents a more economical solution for healthcare systems [23].
The Growing Demand for Ferric Carboxymaltose
The demand for FCM has been steadily rising, driven by several factors:
- Rising Incidence of Iron Deficiency Anemia: IDA remains a global health challenge, especially in developing countries like India, where it disproportionately affects women, children, and pregnant women [24]. According to the World Health Organization (WHO), iron deficiency is the most common and widespread nutritional disorder globally. This has spurred an increase in demand for more efficient treatments like FCM [25].
- Increased Awareness: As awareness about the benefits of FCM grows, both among healthcare professionals and patients, the preference for this intravenous iron formulation is shifting from traditional oral iron supplements [26,27]. Its efficacy, combined with fewer ferric carboxymaltose side effects, makes it an attractive alternative for those with severe or chronic iron deficiency [28].
- Supportive Regulatory Environment: In India, favorable government policies supporting pharmaceutical innovation and the production of high-quality APIs have facilitated the growth of the FCM market [29,30,31]. This environment has allowed companies like WBCIL to continue enhancing their manufacturing capabilities and expand their global reach.
- Global Market Reach: WBCIL ferric carboxymaltose formulations are not only in high demand domestically but are also gaining traction in international markets. The company’s commitment to high standards of manufacturing and regulatory compliance ensures that its products meet the needs of healthcare providers worldwide.
Conclusion: The Future of Ferric Carboxymaltose and WBCIL’s Impact
Ferric carboxymaltose represents a significant advancement in the treatment of iron deficiency anemia, offering improved patient outcomes, a better safety profile, and convenience over traditional iron supplements. With its high efficacy and growing global demand, FCM is poised to play a central role in the management of IDA.
WBCIL, as a leader in the development and manufacturing of FCM, continues to drive innovation in the pharmaceutical industry. Through its R&D efforts, the company not only contributes to the scientific understanding of FCM but also ensures that patients worldwide benefit from its groundbreaking treatment. As WBCIL expands its research, production capabilities, and market presence, the company is set to remain at the forefront of addressing the global challenge of iron deficiency anemia.
With its commitment to quality, innovation, and patient well-being, WBCIL’s role in the future of iron supplementation remains a cornerstone of the pharmaceutical landscape. As the world continues to face the challenges of iron deficiency, ferric carboxymaltose, and the pioneering efforts of companies like WBCIL, offer hope for millions of people seeking effective solutions to improve their health and quality of life.
1. Lyseng-Williamson KA, Keating GM. Ferric carboxymaltose: a review of its use in iron-deficiency anaemia. Drugs. 2009;69(6):739-56. doi: 10.2165/00003495-200969060-00007. PMID: 19405553. https://pubmed.ncbi.nlm.nih.gov/19405553/
2. https://go.drugbank.com/drugs/DB08917
3. https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_203565.pdf
4. Keating GM. Ferric carboxymaltose: a review of its use in iron deficiency. Drugs. 2015 Jan;75(1):101-27. doi: 10.1007/s40265-014-0332-3. PMID: 25428711. https://pubmed.ncbi.nlm.nih.gov/25428711/
5. Mentz RJ, Garg J, Rockhold FW, Butler J, De Pasquale CG, Ezekowitz JA, Lewis GD, O’Meara E, Ponikowski P, Troughton RW, Wong YW, She L, Harrington J, Adamczyk R, Blackman N, Hernandez AF; HEART-FID Investigators. Ferric Carboxymaltose in Heart Failure with Iron Deficiency. N Engl J Med. 2023 Sep 14;389(11):975-986. doi: 10.1056/NEJMoa2304968. Epub 2023 Aug 26. PMID: 37632463. https://pubmed.ncbi.nlm.nih.gov/37632463/
6. Wolf M, Rubin J, Achebe M, et al. Effects of Iron Isomaltoside vs Ferric Carboxymaltose on Hypophosphatemia in Iron-Deficiency Anemia: Two Randomized Clinical Trials. JAMA. 2020;323(5):432–443. doi:10.1001/jama.2019.22450. https://jamanetwork.com/journals/jama/fullarticle/2760391
7. https://www.cda-amc.ca/ferric-carboxymaltose
8. https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/ferric-carboxymaltose
9. https://www.webmd.com/drugs/2/drug-164825/ferric-carboxymaltose-intravenous/details
10. https://www.nps.org.au/radar/articles/ferric-carboxymaltose-ferinject-for-iron-deficiency-anaemia
11. https://www.ipc.gov.in/images/Ferric_Carboxymaltose.pdf
12. https://hhs.iowa.gov/media/391/download?inline
13. https://www.wbcil.com/api-fine-chemicals-nutraceutical/iron/ferric-carboxymaltose/
14. https://www.ijfmr.com/papers/2023/6/9309.pdf
15. https://www.ijsr.net/getabstract.php?paperid=SR231006130120
16. https://www.ptinews.com/press-release/wbcil–a-legacy-of-innovation-and-excellence-in-pharmaceutical-manufacturing/1684907
17. https://www.thehindu.com/business/wbcil-secures-ninth-patiet-for-ferric-maltol/article68411355.ece
18. Froessler, B., Collingwood, J., Hodyl, N.A. et al. Intravenous ferric carboxymaltose for anaemia in pregnancy. BMC Pregnancy Childbirth 14, 115 (2014). https://doi.org/10.1186/1471-2393-14-115. https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/1471-2393-14-115
19. https://www.accessdata.fda.gov/drugsatfda_docs/psg/FERRIC%20CARBOXYMALTOSE_injection_RLD%20203565_RC04-16.pdf
20. Cirillo, L., Somma, C., Allinovi, M. et al. Ferric carboxymaltose vs. ferrous sulfate for the treatment of anemia in advanced chronic kidney disease: an observational retrospective study and cost analysis. Sci Rep 11, 7463 (2021). https://doi.org/10.1038/s41598-021-86769-z. https://www.nature.com/articles/s41598-021-86769-z
21. https://www.seslhd.health.nsw.gov.au/sites/default/files/documents/Medicine%20Guideline%20-%20Ferric%20Carboxymaltose%20%28Ferinject%29%20-%20Iron.pdf
22. Friedrisch JR, Cançado RD. Intravenous ferric carboxymaltose for the treatment of iron deficiency anemia. Rev Bras Hematol Hemoter. 2015 Nov-Dec;37(6):400-5. doi: 10.1016/j.bjhh.2015.08.012. Epub 2015 Oct 14. PMID: 26670403; PMCID: PMC4678908. https://pmc.ncbi.nlm.nih.gov/articles/PMC4678908/
23. https://pdf.ipinnovative.com/pdf/15870
24. Jane E. Onken, David B. Bregman, Robert A. Harrington, David Morris, John Buerkert, Douglas Hamerski, Hussain Iftikhar, Roberto Mangoo-Karim, Edouard R. Martin, Carlos O. Martinez, George Edward Newman, Wajeh Y. Qunibi, Dennis L. Ross, Bhupinder Singh, Mark T. Smith, Angelia Butcher, Todd A. Koch, Lawrence T. Goodnough, Ferric carboxymaltose in patients with iron-deficiency anemia and impaired renal function: the REPAIR-IDA trial, Nephrology Dialysis Transplantation, Volume 29, Issue 4, April 2014, Pages 833–842, https://doi.org/10.1093/ndt/gft251
25. Pasricha SR, Mwangi MN, Moya E, Ataide R, Mzembe G, Harding R, Zinenani T, Larson LM, Demir AY, Nkhono W, Chinkhumba J, Simpson JA, Clucas D, Stones W, Braat S, Phiri KS. Ferric carboxymaltose versus standard-of-care oral iron to treat second-trimester anaemia in Malawian pregnant women: a randomised controlled trial. Lancet. 2023 May 13;401(10388):1595-1609. doi: 10.1016/S0140-6736(23)00278-7. Epub 2023 Apr 21. PMID: 37088092; PMCID: PMC10193370. https://pubmed.ncbi.nlm.nih.gov/37088092/
26. Khatib MN, Sinha AP, Gaidhane S, Upadhyay S, Waghmare N, Anil A, Saxena D, Sawleshwarkar S, Simkhada PP, Gaidhane A, Quazi ZS. Effect of IV ferric carboxy maltose for moderate/severe anemia: a systematic review and meta-analysis. Front Med (Lausanne). 2024 Feb 9;11:1340158. doi: 10.3389/fmed.2024.1340158. PMID: 38405188; PMCID: PMC10884292. https://pubmed.ncbi.nlm.nih.gov/38405188/
27. Scott, L.J. Ferric Carboxymaltose: A Review in Iron Deficiency. Drugs 78, 479–493 (2018). https://doi.org/10.1007/s40265-018-0885-7. https://link.springer.com/article/10.1007/s40265-018-0885-7
28. https://www.medicalnewstoday.com/articles/injectafer#side-effects
29. Mentz RJ, Ambrosy AP, Ezekowitz JA, Lewis GD, Butler J, Wong YW, De Pasquale CG, Troughton RW, O’Meara E, Rockhold FW, Garg J, Samsky MD, Leloudis D, Dugan M, Mundy LM, Hernandez AF; HEART-FID Trial Investigators. Randomized Placebo-Controlled Trial of Ferric Carboxymaltose in Heart Failure With Iron Deficiency: Rationale and Design. Circ Heart Fail. 2021 May;14(5):e008100. doi: 10.1161/CIRCHEARTFAILURE.120.008100. Epub 2021 May 18. PMID: 34003690; PMCID: PMC8136455. https://pubmed.ncbi.nlm.nih.gov/34003690/
30. Korczowski, B., Farrell, C., Falone, M. et al. Safety, pharmacokinetics, and pharmacodynamics of intravenous ferric carboxymaltose in children with iron deficiency anemia. Pediatr Res 94, 1547–1554 (2023). https://doi.org/10.1038/s41390-023-02644-9
31. https://med.virginia.edu/pediatrics/wp-content/uploads/sites/237/2015/12/May17_Ferric-carboxymaltose_PedPharmaco.pdf
Ferric Carboxymaltose (FCM) is an advanced intravenous (IV) iron therapy used to treat iron deficiency anemia. Unlike oral iron supplements, which rely on gastrointestinal absorption, FCM delivers iron directly into the bloodstream, making it ideal for patients with absorption issues or those who cannot tolerate oral treatments. It replenishes iron stores efficiently, helping the body produce hemoglobin, which is essential for carrying oxygen throughout the body.
Ferric Carboxymaltose offers several advantages over oral iron supplements, such as:
- Faster absorption: Delivered directly to the bloodstream, bypassing the digestive system.
- Fewer side effects: Minimal gastrointestinal discomfort, such as nausea or constipation, which are common with oral iron.
- Convenience: Requires fewer doses, often administered in a single or limited number of infusions.
These benefits make FCM particularly effective for patients with malabsorption issues, chronic diseases, or those requiring quick iron restoration.
In conditions like chronic kidney disease (CKD) or heart failure, iron deficiency is common due to chronic inflammation and impaired iron absorption. FCM offers the following benefits:
- Improves energy levels and reduces symptoms of fatigue.
- Helps in managing anemia without relying heavily on erythropoietin-stimulating agents (ESAs).
- Enhances exercise capacity and overall quality of life for heart failure patients.
- Provides a safe and effective alternative for patients unable to tolerate oral iron therapy.
Compared to other IV iron formulations, Ferric Carboxymaltose is superior due to its:
- Safety profile: Lower risk of allergic reactions, including anaphylaxis.
- Higher dosage per infusion: Can deliver up to 1,000 mg of iron in one session, reducing the need for multiple visits.
- Stability: Its advanced formulation minimizes free iron, reducing oxidative stress and enhancing patient safety.
This makes FCM the preferred choice for healthcare providers and patients alike.
Ferric Carboxymaltose is well-tolerated, but like any medication, it may cause mild side effects in some patients. These include:
- Temporary headaches or dizziness.
- Mild nausea or vomiting.
- Local reactions like pain or bruising at the injection site.
Serious allergic reactions are rare due to FCM’s stable formulation, but patients are typically monitored during administration to ensure safety.
West Bengal Chemical Industries Limited (WBCIL) maintains the highest standards in producing Ferric Carboxymaltose APIs by:
- Following CGMP (Current Good Manufacturing Practices) and adhering to ISO certifications.
- Utilizing patented manufacturing processes to ensure purity and potency.
- Conducting rigorous quality control tests throughout production, ensuring global compliance with pharmacopoeial standards like USP, BP, and EP.
This dedication ensures that WBCIL’s Ferric Carboxymaltose is safe, effective, and trusted by healthcare providers worldwide.
Yes, Ferric Carboxymaltose is considered safe and effective for pregnant women suffering from iron deficiency anemia, especially during the second and third trimesters. It helps restore iron levels quickly, reducing risks to both the mother and the baby, such as premature delivery or low birth weight. However, its use should always be guided by a healthcare professional.
WBCIL stands out due to its:
- Pioneering expertise in API manufacturing since 1962.
- Commitment to innovation through continuous research and development.
- Stringent adherence to global quality standards, ensuring superior purity and efficacy.
- Global reach, supplying high-quality APIs to pharmaceutical companies worldwide.
These factors position WBCIL as a trusted leader in the production of Ferric Carboxymaltose.
Yes, Ferric Carboxymaltose is highly cost-effective due to its:
- Single or fewer infusion requirements, reducing hospital visits and associated costs.
- Rapid improvement in symptoms, minimizing the need for additional treatments.
- Long-lasting benefits, which improve patient compliance and overall health outcomes.
By addressing anemia efficiently, FCM also reduces the indirect costs of untreated anemia, such as lost productivity and increased healthcare expenses.
Yes, West Bengal Chemical Industries Limited (WBCIL) actively exports Ferric Carboxymaltose (FCM) to the MENA (Middle East and North Africa) region, among other international markets. The MENA region has a growing demand for advanced iron supplementation therapies due to the high prevalence of iron deficiency anemia in these countries, particularly among women, children, and individuals with chronic conditions like kidney disease or heart failure.
