-
Product Name:
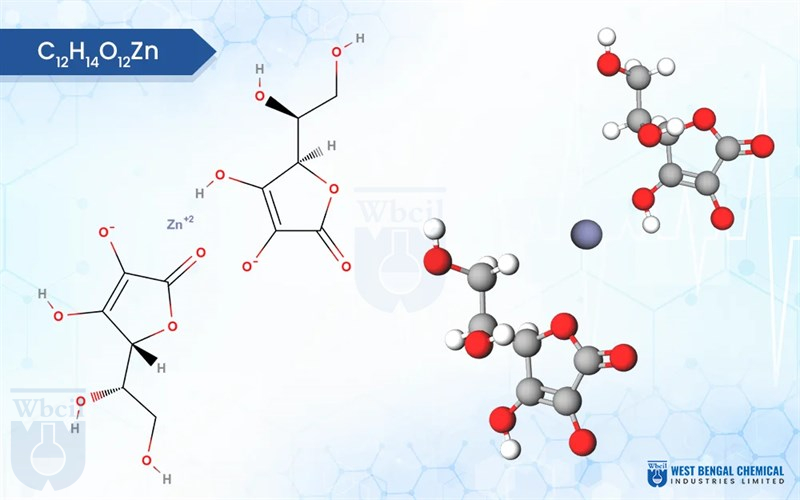
Zinc Ascorbate
-
Molecular Formula:
C12H14O12Zn
-
Molecular Weight:
415.6 g/mol
-
CAS No.:
331242-75-2
-
HSN Code:
29362700
-
CID Code:
91667835
-
Shelf Life:
3 years - 20°C powder
-
DrugBank ID
DB14485
-
ChemSpider ID
34988958
-
UNII No.
9TI35313XW
- USP
- IUPAC Names
- Synonyms
- MSDS
USP of Zinc Ascorbate
- Zinc ascorbate offer superior antioxidant benefits, which help to combat free radicals and reduces oxidative damage.
- Zinc ascorbates provide a dual-action immune support, helping the body fight off infections and infections and illnesses.
IUPAC Names of Zinc Ascorbate
zinc;(2R)-2-[(1S)-1,2-dihydroxyethyl]-3-hydroxy-5-oxo-2H-furan-4-olate
Synonyms of Zinc Ascorbate
- Zinc ascorbate
- L-Ascorbic acid, zinc salt
- Zinc ascorbate [WHO-DD]
- (-)-Ascorbic acid, zinc salt
- UNII-9TI35313XW
- zinc;(2R)-2-[(1S)-1,2-dihydroxyethyl]-3-hydroxy-5-oxo-2H-furan-4-olate
MSDS of Zinc Ascorbate
Download MSDS PDF- Inhalation: Move exposed person to fresh air. If not breathing, seek immediate medical attention. If breathing is irregular or if respiratory arrest occurs, provide artificial respiration or oxygen by trained personnel and seek medical attention.
- Ingestion: Do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Seek medical attention.
- Skin Contact: Remove contaminated clothing and shoes and immediately flush skin with plenty of water for at least 15 minutes. Wash clothing before reuse. Clean shoes thoroughly before reuse. If irritation persists, seek medical attention.
- Eye Contact: Check for and remove any contact lenses. Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Seek immediate medical attention.
- Flammability of the Product: May be combustible at high temperature.
- Auto-Ignition Temperature: Not available.
- Flash Points: Not available.
- Flammable Limits: These products are carbon oxides (CO, CO2), nitrogen oxides (NO, NO2…). Some metallic oxides.
- Products of Combustion: Slightly flammable to flammable in presence of heat. Non-flammable in presence of shocks.
- Fire Hazards in Presence of Various Substances: Slightly explosive in presence of open flames and sparks. Non-explosive in presence of shocks.
- Fire Fighting Media and Instructions:
SMALL FIRE: Use DRY chemical powder.
LARGE FIRE: Use water spray, fog or foam. Do not use water jet. - Special Remarks on Fire Hazards: Fire is possible at elevated temperatures.
- Special Remarks on Explosion Hazards: Fine dust dispersed in air in sufficient concentrations, and in the presences of an ignition source is a potential dust explosion hazard.
- Engineering Controls: Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below Recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit.
- Personal Protection: Safety glasses. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves.
- Personal Protection in Case of a Large Spill: Splash goggles. Full suit. Dust respirator. Boots. Gloves. A selfcontained breathing apparatus should be used to avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product.
- Exposure Limits: Not available.
- Appearance Form: Off white to yellowish powder
- pH (of 2% w/v aqueous solution): No data available
- Melting point/freezing point: No data available
- Initial boiling point and boiling range: No data available
- Sublimation Temperature: No data available
- Other safety information: No data available
Description of Zinc Ascorbate
Zinc ascorbate, a mineral supplement combining zinc and vitamin C, boasts unique characteristics. Physically, it appears as a white, odorless powder with a fine texture. Unlike pure vitamin C (ascorbic acid), zinc ascorbate is hygroscopic, meaning it readily absorbs moisture from the air. This property necessitates storage in airtight containers to maintain its powdery form. Chemically, zinc ascorbate is a coordination complex.
The zinc ion (Zn²⁺) forms bonds with two ascorbate anions (C₆H⁷O₆⁻), the ionized form of vitamin C. This bond between the mineral and vitamin enhances stability compared to pure ascorbic acid, making it less prone to degradation in light and moisture. As a leading API manufacturer, WBCIL prioritizes strict CGMP and ISO quality control measures throughout the production process. The prolonged experience from 1962 ensures consistent potency, purity, and safety in every dose of WBCIL’s zinc ascorbate.
Application of Zinc Ascorbate
Pharmaceutical Industry
- Immune-boosting supplements: Zinc ascorbate can be used in formulating immune support supplements, especially during cold and flu seasons.
- Antioxidant formulations: It can be incorporated into antioxidant supplements to combat oxidative stress and support overall health.
Nutraceutical Industry
- Functional foods: Zinc ascorbate can be added to fortified foods and beverages to enhance their nutritional value and provide immune support 2.
- Sports nutrition: It can be used in pre-workout or recovery supplements to support immune function and reduce oxidative stress in athletes.
Cosmetic and Skincare Industry
- Anti-aging products: The antioxidant properties of zinc ascorbate make it suitable for use in anti-aging creams and serums to combat free radical damage to the skin.
- Wound healing formulations: Its dual benefits can be utilized in products designed to support skin repair and regeneration.