Iron Deficiency Solution- The Science Behind Ferric Carboxymaltose
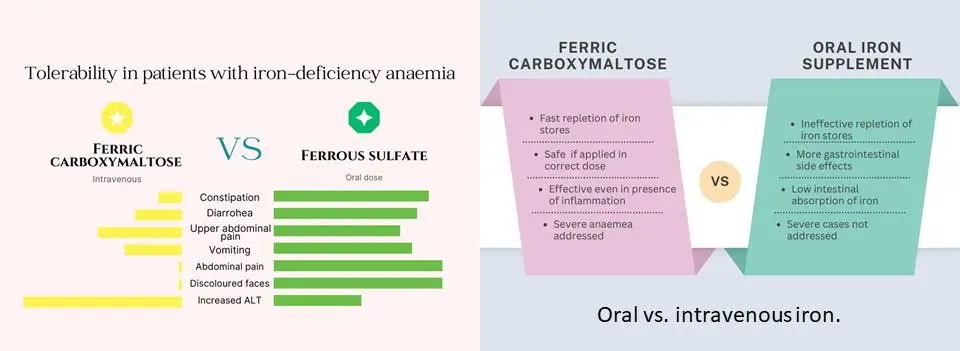
Let’s face it, oral iron supplements can be the bane of many people’s existence. While they aim to be warriors against iron deficiency solution, they often leave a trail of digestive destruction in their wake 1. This is where ferric carboxymaltose swoops in, offering a peaceful (well, mostly peaceful) alternative for those with sensitive stomachs 2 3.
The Usual Suspects:
The most common culprits behind the digestive woes caused by oral iron are:
- Constipation: Iron can act like a tiny traffic jam in your gut, slowing down the movement of things and leaving you feeling backed up.
- Nausea and Vomiting: Iron can irritate your stomach lining, causing nausea and even vomiting in some people.
- Stomach Pain and Heartburn: Similar to nausea, iron can trigger discomfort and a burning sensation in the stomach.
Iron by the Bypass:
Ferric carboxymaltose bypasses this digestive battlefield altogether. Since it’s administered intravenously, it enters your bloodstream directly, avoiding any potential irritation to your stomach and intestines. This makes it a godsend for people who experience these unpleasant side effects with oral iron 4 5 6.
How Does Ferric Carboxymaltose Work for iron deficiency solution?
Ferric carboxymaltose (FCM) tackles iron deficiency with a unique approach. Unlike oral iron that relies on the sometimes-fickle digestive system, FCM is an injectable iron complex. Ferric carboxymaltose mode of action delivers iron directly into the bloodstream, bypassing absorption issues in the gut 7 8. The sugar molecule carboxymaltose acts as a carrier, slowly releasing iron to be taken up by cells of the immune system. These cells then distribute iron to essential proteins like ferritin for storage and transferrin for transport throughout the body. This controlled release minimizes the risk of iron overload and allows for efficient replenishment of iron stores, ultimately promoting red blood cell production. These red blood cells are the oxygen carriers in your body, and with more of them, you’ll feel energized and revitalized 9 10 11 12 13.

The Chemical Structure:
Ferric carboxymaltose structure is a unique complex molecule in pharmacology. The core element is ferric iron (Fe3+), which is essential for many biological processes. This iron is bound to carboxymaltose, a complex carbohydrate polymer. Carboxymaltose is a modified version of maltose, a sugar molecule, with additional carboxylic acid groups attached. The specific structure of carboxymaltose allows it to encapsulate multiple ferric ions, forming a colloidal iron (III) hydroxide complex. This complex is crucial because it facilitates the controlled release of iron within the body while minimizing the risk of iron overload, a potential side effect of free iron 14 15 16 17. Ferric carboxymaltose molecular weight (average) is around 788 g/mol.
Beyond Relief: Enhanced Absorption
Ferric carboxymaltose benefits go beyond just avoiding digestive discomfort. Oral iron supplements often have a lower absorption rate, meaning your body might not be fully utilizing the iron you’re taking. Ferric carboxymaltose uses, on the other hand, boasts a higher bioavailability, meaning your body can absorb a greater percentage of the iron it delivers 18 19 20. This translates to a faster and more effective way to replenish your iron stores. An iron infusion (FCM) was compared to iron pills (ferrous sulfate) for treating anemia in chronic kidney disease. FCM worked better, raising iron levels and reducing the need for other medications. Though initially more expensive, FCM led to lower overall treatment costs 21
Ferric carboxymaltose (FCM) shines when it comes to bioavailability, outperforming common iron supplements 22. Here’s a breakdown:
- Oral Iron: The go-to for iron deficiency, but absorption suffers due to gut health, inflammation, and dietary factors. Ferrous sulphate, a popular choice, has only about 10-15% bioavailability.
- Intravenous (IV) Iron: Another injectable option, iron sucrose offers better absorption (30-50%) than oral iron. However, FCM might have an edge. Studies suggest FCM may replenish iron stores and haemoglobin levels faster than iron sucrose.
- Ferric Carboxymaltose (FCM): Ferric carboxymaltose intravenous injectable iron boasts a whopping 90% estimated bioavailability. This translates to efficient iron delivery directly into the bloodstream for immediate use 23 24.
Not Without Its Fight:
Like any superhero, it isn’t without its challenges, ferric carboxymaltose adverse effects are present. It’s a prescription medication that requires a healthcare professional to administer it. There can also be ferric carboxymaltose side effects, although generally mild, like metallic taste or headache.
Ferric Carboxymaltose in Anaemia:
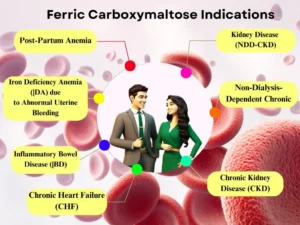
While iron deficiency anaemia in pregnancy can harm both mother and baby, ferric carboxymaltose use in pregnancy requires careful consideration 31. Although some studies suggest it might be safe and effective, particularly in the second and third trimesters, there’s limited research on its effects on the developing foetus 32 33. Due to potential risks, doctors will only prescribe ferric carboxymaltose if the benefits of correcting iron deficiency clearly outweigh potential risks to the foetus. This medication is typically reserved for situations where oral iron supplements are ineffective or poorly tolerated.
Ferric Carboxymaltose in pharmacology:
Ferric carboxymaltose in pharmacology is used to address iron deficiency anaemia. Unlike oral iron supplements, it is particularly beneficial for patients who experience intolerance or inadequate response to those 34. Additionally, ferric carboxymaltose dosage offers an alternative for adults with chronic kidney disease not on dialysis and for specific cases of heart failure where iron deficiency limits exercise capacity. This medication functions by delivering controlled amounts of iron into the body’s iron storage system and transport proteins. It’s typically administered intravenously, either as a quick undiluted ferric carboxymaltose injection for smaller doses or as a diluted short infusion for larger doses 35.
A study investigated iron infusions (FCM) as a treatment for anaemia in children with chronic kidney disease. The results were promising! FCM safely improved haemoglobin and iron levels in these children, with minimal side effects. This suggests FCM could be a valuable approach for managing anemia in this patient population. 36.
Iron infusions (FCM) were more effective than iron tablets for treating anaemia after childbirth in Tanzania. This suggests FCM could be a valuable tool in low-income settings. More research is needed to confirm safety, cost, and long-term effects in other areas and patient groups 37.
Ferric Carboxymaltose warnings:
A crucial ferric carboxymaltose contraindication is a known allergy to FCM or similar iron injection products 38. Additionally, individuals with iron overload or chronically high iron levels should follow ferric carboxymaltose precaution. It’s also important to disclose pre-existing liver disease to a doctor before ferric carboxymaltose administration, as it may require additional monitoring.
Is Ferric Carboxymaltose Right for You?
If you’re struggling with iron deficiency anaemia and oral supplements haven’t been effective, talk to your doctor about ferric carboxymaltose dosing. It might be the iron fist you need to conquer your anaemia and get back to feeling your best.
Conclusion:
In conclusion, ferric carboxymaltose offers a valuable alternative for treating iron deficiency anemia, particularly in cases where oral iron is ineffective or poorly tolerated. This injectable iron therapy effectively replenishes iron stores and improves red blood cell production. While potential side effects exist, they are generally mild and well-managed. Ferric carboxymaltose provides a safe and efficient approach to correcting iron deficiency and improving patient outcomes.
Why choose us?
West Bengal Chemical Industries Limited is the patent holder of Ferric Carboxymaltose with less side-effects obtained cost effectively.WBCIL, a leading ferric carboxymaltose API supplier and exporter in India, provides top-quality ferric carboxymaltose supplements and powder, ensuring reliable and effective solutions for iron supplementation needs. Ferric carboxymaltose manufacturers in India, including West Bengal Chemical Industries Limited (WBCIL), ensure the highest standards of purity and effectiveness in their products. Contact us for more details!
-
Arici AM, Kumral Z, Gelal A, Akdeniz B. Fatal anaphylactic reaction due to ferric carboxymaltose: A case report. Anatol J Cardiol. 2020 Aug;24(2):115-117. doi: 10.14744/AnatolJCardiol.2020.38996. PMID: 32749249; PMCID: PMC7460686. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7460686/
-
Ferric Carboxymaltose, Memorial Sloan Kettering Cancer Center https://www.mskcc.org/cancer-care/patient-education/medications/adult/ferric-carboxymaltose
-
Melvin H. Seid, Antoinette Mangione, Thomas G. Valaoras, Lowell B. Anthony, Charles F. Barish; Safety Profile of Iron Carboxymaltose, a New High Dose Intravenous Iron in Patients with Iron Deficiency Anemia.. Blood2006; 108 (11): 3739. https://ashpublications.org/blood/article/108/11/3739/127367/Safety-Profile-of-Iron-Carboxymaltose-a-New-High
-
Wolf M, Rubin J, Achebe M, et al. Effects of Iron Isomaltoside vs Ferric Carboxymaltose on Hypophosphatemia in Iron-Deficiency Anemia: Two Randomized Clinical Trials. 2020;323(5):432–443. doi:10.1001/jama.2019.22450. https://jamanetwork.com/journals/jama/fullarticle/2760391
-
Bregman DB, Goodnough LT. Experience with intravenous ferric carboxymaltose in patients with iron deficiency anemia. Therapeutic Advances in Hematology. 2014;5(2):48-60. doi:1177/2040620714521127
-
Kant, Shashi1; Kaur, Ravneet2,; Ahamed, Farhad3; Singh, Archana4; Malhotra, Sumit5; Kumar, Rakesh2. Effectiveness of Intravenous Ferric Carboxymaltose in Improving Hemoglobin Level among Postpartum Women with Moderate-to-Severe Anemia at a Secondary Care Hospital in Faridabad, Haryana – An Interventional Study. Indian Journal of Public Health 64(2):p 168-172, Apr–Jun 2020. | DOI: 10.4103/ijph.IJPH_85_19 https://journals.lww.com/ijph/fulltext/2020/64020/effectiveness_of_intravenous_ferric_carboxymaltose.13.aspx
-
Kanani P, Shah S, Malhotra S. Anaphylactic reaction to ferric carboxymaltose: A case series. jpadr [Internet]. 2022Dec.1 [cited 2024May27];3(4):35-8. https://www.jpadr.com/index.php/jpadr/article/view/111
-
Ferric Carboxymaltose https://www.wikidoc.org/index.php/Ferric_Carboxymaltose
-
ferric carboxymaltose https://www.myactivehealth.com/hwcontent/content/multum/d07305a1.html
-
Jessica Caporuscio, PharmDAll About Injectafer, 2021, https://www.healthline.com/health/drugs/injectafer
-
Ferric carboxymaltose (Ferinject▼): risk of symptomatic hypophosphataemia leading to osteomalacia and fractures, Medicines and Healthcare products Regulatory Agency, Drug Safety Update, volume 14, issue 4: November 2020: 3. https://www.gov.uk/drug-safety-update/ferric-carboxymaltose-ferinject-risk-of-symptomatic-hypophosphataemia-leading-to-osteomalacia-and-fractures
-
Ferric carboxymaltose and low blood phosphorous, Australian government, Department of Health and Aged Care, Therapeutic Goods Administration, https://www.tga.gov.au/news/safety-updates/ferric-carboxymaltose-and-low-blood-phosphorous
-
Rognoni, C., Venturini, S., Meregaglia, M. et al.Efficacy and Safety of Ferric Carboxymaltose and Other Formulations in Iron-Deficient Patients: A Systematic Review and Network Meta-analysis of Randomised Controlled Trials. Clin Drug Investig 36, 177–194 (2016). https://doi.org/10.1007/s40261-015-0361-z
-
Charmila A, Natarajan S, Chitra TV, Pawar N, Kinjawadekar S, Firke Y, Murugesan U, Yadav P, Ohri N, Modgil V, Rodge A, Swami OC. Efficacy and Safety of Ferric Carboxymaltose in the Management of Iron Deficiency Anemia: A Multi-Center Real-World Study from India. J Blood Med. 2022;13:303-313 https://doi.org/10.2147/JBM.S361210
-
Garcia-Ortega P, Jimenez-Lozano I, Cruz Á, Polo AF, Lopez M, Ariceta G. Safety and effectiveness of ferric carboxymaltose intravenous therapy in pediatric patients with chronic kidney disease. Front Pediatr. 2022 Oct 6;10:967233. doi: 10.3389/fped.2022.967233. PMID: 36275063; PMCID: PMC9582777. https://pubmed.ncbi.nlm.nih.gov/36275063/
-
Increases in Fibroblast Growth Factor 23 During Treatment With Ferric Carboxymaltose: Potential Adverse Effects on the Heart and Kidneys, Journal of Cardiac Failure, VOLUME 29, ISSUE 2, P229-231, FEBRUARY 2023 https://onlinejcf.com/article/S1071-9164(22)01205-2/fulltext
-
Children Korczowski, B., Farrell, C., Falone, M. et al. Safety, pharmacokinetics, and pharmacodynamics of intravenous ferric carboxymaltose in children with iron deficiency anemia. Pediatr Res 94, 1547–1554 (2023). https://doi.org/10.1038/s41390-023-02644-9
-
Gilmartin, C.E., Hoang, T., Cutts, B.A. and Leung, L. (2018), Retrospective cohort study comparing the adverse reactions and efficacy of intravenous iron polymaltose with ferric carboxymaltose for iron deficiency anemia. Int J Gynecol Obstet, 141: 315-320. https://doi.org/10.1002/ijgo.12476
-
Cirillo, L., Somma, C., Allinovi, M. et al.Ferric carboxymaltose vs. ferrous sulfate for the treatment of anemia in advanced chronic kidney disease: an observational retrospective study and cost analysis. Sci Rep 11, 7463 (2021). https://doi.org/10.1038/s41598-021-86769-z
-
Injectafer (ferric carboxymaltose), MEDICALNEWSTOSAY https://www.medicalnewstoday.com/articles/injectafer#about
-
Friedrisch JR, Cançado RD. Intravenous ferric carboxymaltose for the treatment of iron deficiency anemia. Rev Bras Hematol Hemoter. 2015 Nov-Dec;37(6):400-5. doi: 10.1016/j.bjhh.2015.08.012. Epub 2015 Oct 14. PMID: 26670403; PMCID: PMC4678908. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4678908/
-
Thanusubramanian H, Patil N, Shenoy S, Bairy KL, Sarma Y. Adverse reactions of ferric carboxymaltose. J Clin Diagn Res. 2014 Oct;8(10):HD01-2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4253187/
-
Injectafer side effects: What you should know, MEDICALNEWSTODAY https://www.medicalnewstoday.com/articles/drugs-injectafer-side-effects
-
Sridhara, S. and Vilakkathala, R. and Prabhu, R. and Saraf, K. Delayed Hypersensitivity with Ferric Carboxymaltose, Journal of Clinical and Diagnostic Research. 2017 Nov, Vol-11(11): FD01-FD02 https://jcdr.net/articles/PDF/10827/25908-CE(RA1)_F(GG)_PF1(PB_AP)_PFA(MJ_SS).pdf
-
Ferric Carboxymaltose, HealthHub, https://www.healthhub.sg/a-z/medications/ferric-carboxymaltose
-
John P. Cunha, DO, FACOEP, INJECTAFER, RxList https://www.rxlist.com/injectafer-drug.htm#description
-
Ferric Carboxymaltose, Comprehensive Pharmacology, 2022 https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/ferric-carboxymaltose
-
Ferric Carboxymaltose (Injectafer), EVERYDAY HEALTH https://www.everydayhealth.com/drugs/ferric-carboxymaltose
-
Pregnancy Froessler, B., Collingwood, J., Hodyl, N.A. et al.Intravenous ferric carboxymaltose for anaemia in pregnancy. BMC Pregnancy Childbirth 14, 115 (2014). https://doi.org/10.1186/1471-2393-14-115
-
Childbirth Vanobberghen, Fiona & Lweno, Omar & Kuemmerle, Andrea & Mwebi, Kwaba & Asilia, Peter & Issa, Amina & Simon, Beatus & Mswata, Sarah & Schmidlin, Sandro & Glass, Tracy & Abdulla, Salim & Daubenberger, Claudia & Tanner, Marcel & Meyer-Monard, Sandrine. (2020). Efficacy and safety of intravenous ferric carboxymaltose compared with oral iron for the treatment of iron deficiency anaemia in women after childbirth in Tanzania: a parallel-group, open-label, randomised controlled phase 3 trial. The Lancet Global Health. 9. 10.1016/S2214-109X(20)30448-4. https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(20)30448-4/fulltext
-
Arastu AH, Elstrott BK, Martens KL, et al. Analysis of Adverse Events and Intravenous Iron Infusion Formulations in Adults With and Without Prior Infusion Reactions. JAMA Netw Open.2022;5(3):e224488. doi:10.1001/jamanetworkopen.2022.4488 https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2790508
Ferric carboxymaltose (FCM) is an intravenous iron replacement product used to treat iron deficiency anemia. It provides a way to bypass the digestive system, directly delivering iron into the bloodstream.
Ferric carboxymaltose is ideal for individuals who cannot tolerate oral iron supplements due to side effects such as gastrointestinal discomfort or those who do not respond adequately to oral iron therapy.
FCM is administered intravenously, either as a quick undiluted injection for smaller doses or as a short infusion for larger doses. It must be given by a healthcare professional
The primary benefits include avoiding gastrointestinal side effects, higher bioavailability, and more efficient replenishment of iron stores. FCM can rapidly increase iron levels and improve symptoms of anemia.
Common side effects include a metallic taste, headache, dizziness, and sometimes a mild allergic reaction. Serious side effects are rare but may include low blood pressure and severe allergic reactions.
Many patients experience an improvement in their anemia symptoms within a few days to weeks after the infusion, with significant increases in hemoglobin and iron levels observed within a month.
The risk of iron overload is minimized due to the controlled release of iron from the carboxymaltose complex. However, individuals with pre-existing iron overload conditions should avoid FCM.
