The Science and Significance of Zinc Salts in Human Health and Industry
Zinc, a vital trace mineral, plays a crucial role in numerous biological functions. When combined with acids, it forms zinc salts, which have a wide range of applications, from industrial processes to healthcare. This blog delves into the world of zinc salts, exploring their properties, uses, and the importance of choosing the right zinc supplement.
Zinc Deficiency: A Global Health Concern
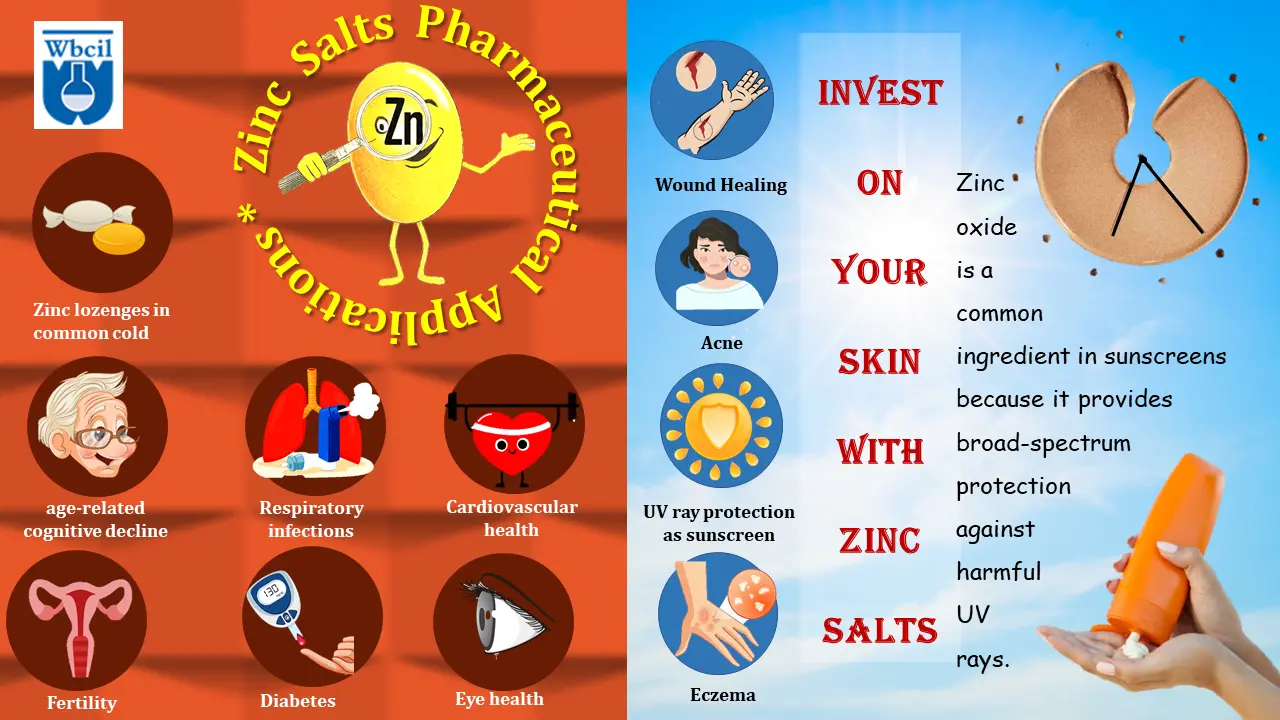
Zinc deficiency, a common nutritional deficiency worldwide, can have significant health consequences. This condition occurs when the body doesn’t absorb enough zinc from the diet or loses too much through certain medical conditions [1]. Symptoms of zinc deficiency can vary widely, but often include impaired immune function, delayed growth and development, skin problems, hair loss, and diarrhea. Zinc deficiency can cause skin rashes that may initially resemble eczema [2]. If left untreated, zinc absorption deficiency can lead to more severe health issues, such as impaired wound healing, decreased fertility, and even increased risk of certain infections [3].

The Chemistry of Zinc Salts
Zinc salts are chemical compounds formed when zinc metal reacts with an acid. Zinc commonly exists in nature as zinc sulphide, zinc carbonate, zinc chromate, or zinc oxide, zinc aspartate, zinc ascorbate, zinc citrate, zinc propionate, zinc acetate [10]. Zinc salts are crystalline solids with varying colours and solubilities, often used in industrial applications and dietary supplements. The most common zinc salts include:
- Zinc chloride (ZnCl₂): A highly soluble, colourless salt used in water treatment, textile manufacturing, and as a catalyst in chemical reactions [11].
- Zinc sulfate (ZnSO₄): A white crystalline powder used in fertilizers, animal feed, and as a mordant in dyeing. It’s also a common ingredient in zinc supplements [12].
- Zinc gluconate (Zn(C₆H₁₁O₇)₂): A less irritating form of zinc used in oral supplements and topical skin care products [13].
- Zinc oxide (ZnO): A white powder used in sunscreens, ointments, and as a pigment in paints and ceramics [14].
The Importance of Zinc in Human Health
Zinc is an essential nutrient involved in various bodily functions, including:
- Immune system: Zinc is crucial for the proper functioning of immune cells, helping to fight infections.
- Growth and development: Zinc is required for cell growth and division, making it essential for children’s development and wound healing [15].
- Protein synthesis: Zinc is a cofactor for many enzymes involved in protein synthesis.
- Sensation: Zinc plays a role in taste perception and may also be involved in hearing and vision [16].
- Antioxidant activity: Zinc can help protect cells from damage caused by free radicals.
- Defeating cold: Studies suggest that zinc lozenges may potentially shorten the duration of common cold symptoms, but further research is needed to confirm its effectiveness and safety [17].
Zinc: A Versatile Element in Human Health and Industry
Zinc is essential for immune function, growth and development, protein synthesis, and sensation. Zinc supplements deficiency can lead to various health problems, including impaired immune function, growth retardation, and skin issues. Industrially, zinc is a highly versatile element with a wide range of applications. It is used in:
- Galvanization: Zinc coatings are applied to metals to protect them from corrosion [4].
- Battery production: Zinc salt is used in the production of alkaline batteries, which are commonly used in everyday devices [5].
- Pigments and coatings: Zinc oxide, a common zinc compound, is used as a pigment in paints and as a coating for metals [6].
- Rubber manufacturing: Zinc oxide is used as a vulcanizing agent in the production of rubber [7].
- Textile industry: Zinc salts are used as mordants to fix dyes to fabrics [8].
- Corrosion prevention: Zinc salts are highly effective cathodic inhibitors for corrosion prevention in cooling water systems [9].
In addition to its industrial applications, zinc is also used in various medical and pharmaceutical products. Zinc oxide is a common ingredient in sunscreens, ointments, and baby powder. Zinc sulphate is used as a dietary supplement and in the treatment of certain medical conditions.

Zinc Deficiency and Its Consequences
A zinc deficiency can lead to various health problems [18,19], including:
- Impaired immune function
- Growth retardation
- Delayed sexual development
- Skin problems
- Hair loss
- Diarrhoea
Choosing the Right Zinc Supplement
When considering zinc supplementation, it’s essential to choose a product that provides adequate amounts of elemental zinc health benefits, which is the form that the body can absorb. Daily zinc supplementation of 15-30 mg can potentially enhance immunity, blood sugar control, and overall eye, heart, and skin health [20]. Here are some guidelines for selecting a zinc supplement:
- Elemental zinc content: Look for a supplement that provides a recommended daily intake of elemental zinc [21].
- Form of zinc: Zinc gluconate and zinc sulphate are common forms, but zinc picolinate and zinc citrate may have better absorption rates [22].
- Other ingredients: Avoid supplements that contain excessive amounts of fillers or binders [23].
- Consult a healthcare professional: If you have underlying health conditions or are taking medications, consult a doctor before starting zinc supplementation [24].
Caution in Using Zinc Salts
While zinc is an essential mineral for human health, excessive zinc intake can lead to adverse effects. Overconsumption of zinc supplements or exposure to excessive amounts of zinc through industrial processes can cause toxicity. Zinc chloride is a highly toxic substance, causing severe irritation to the eyes and skin, and classified as Toxicity Category I [25]. Symptoms of zinc toxicity may include nausea, vomiting, diarrhoea, abdominal pain, and copper deficiency. It is essential to consult with a healthcare professional before starting zinc supplementation, especially if you have underlying health conditions or are taking medications. Additionally, individuals exposed to zinc in industrial settings should take appropriate precautions to avoid excessive exposure.
Intravenous Zinc: A Medical Treatment
In severe cases of zinc deficiency or when oral supplementation is not effective, intravenous zinc therapy may be necessary. This involves administering zinc directly into the bloodstream, allowing for rapid absorption and delivery to the body’s tissues.
Chelated zinc
It is a highly bioavailable form of the essential mineral zinc, bound to a chelating agent like EDTA. This binding increases zinc’s solubility and absorption, making it more readily available to plants. Chelated zinc like zinc bisglycinate plays a crucial role in various plant processes, including enzyme activation, protein synthesis, and photosynthesis [26].
It is particularly important for crop growth and development, especially in areas with zinc-deficient soils. By providing plants with a readily absorbed source of zinc, chelated zinc can enhance yield, improve crop quality, and increase resistance to stress factors.
Zinc Salts in Industry
Zinc salts have numerous industrial applications, including:
- Zinc sulphate is a common zinc supplement in dry fertilizer formulations, ensuring adequate zinc availability for plant growth.
- Zinc chloride fluxes have been the industry standard for soldering applications for centuries, renowned for their effectiveness and versatility.
- Zinc gluconate is a popular zinc supplement that combines zinc with gluconic acid for enhanced absorption and bioavailability [27].
Zinc acetate is a food preservative that inhibits bacterial growth and prolongs shelf life.
Zinc Salts for Skin Health
Zinc plays a crucial role in wound healing by supporting collagen synthesis and reducing inflammation. Zinc salts, particularly zinc oxide, are commonly used in skincare products due to their beneficial properties. Zinc oxide is a natural mineral that provides broad-spectrum protection against harmful UV rays, making it an essential ingredient in sunscreens.
Zinc oxide-based sunscreens are often non-comedogenic, making them suitable for those with acne-prone skin. It also has anti-inflammatory and antimicrobial properties, which can help soothe irritated skin and reduce acne breakouts. Additionally, zinc oxide can help accelerate wound healing and promote skin regeneration.
Zinc supplements offered by WBCIL
- Zinc Gluconate
- Zinc Bisglycinate
- Zinc Aspartate
- Zinc Ascorbate
- Zinc Citrate
- Zinc Propionate
- Zinc Acetate
- Liposomal Zinc
Liposomal Zinc
Liposomal zinc is a highly bioavailable form of zinc that is encapsulated in protective liposomes for enhanced absorption. It offers superior benefits for immune health, wound healing, and overall well-being.
WBCIL: A Trusted Manufacturer of Zinc Supplements
WBCIL is a renowned manufacturer of high-quality zinc supplements. Their products are formulated using pure, elemental zinc and are rigorously tested to ensure safety and efficacy. WBCIL’s commitment to quality and customer satisfaction makes them a trusted choice for those seeking reliable zinc supplementation.
Conclusion
Zinc salts are versatile compounds with a wide range of applications, from industrial processes to human health. Understanding the properties and uses of zinc salts is essential for making informed choices about zinc supplementation and other related products.
By choosing the right zinc supplement and consulting with a healthcare professional, you can ensure that you are meeting your body’s zinc needs and supporting optimal health.
1. https://www.sciencedirect.com/topics/chemical-engineering/zinc-salts#:~:text=Zinc%20salts%20are%20well%2Dknown,maximize%20the%20effect%20of%20inhibition.
2. Bourne N, Stegall R, Montano R, Meador M, Stanberry LR, Milligan GN. Efficacy and toxicity of zinc salts as candidate topical microbicides against vaginal herpes simplex virus type 2 infection. Antimicrobe Agents Chemother. 2005 Mar;49(3):1181-3. doi: 10.1128/AAC.49.3.1181-1183.2005. PMID: 15728922; PMCID: PMC549274. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC549274/
3. https://archive.epa.gov/pesticides/reregistration/web/pdf/zinc_salt.pdf
4. https://www.cir-safety.org/sites/default/files/zincst092017SLR.pdf
5. Stéphan F, Revuz J. Sels de zinc en dermatologie [Zinc salts in dermatology]. Ann Dermatol Venereol. 2004 May;131(5):455-60. French. doi: 10.1016/s0151-9638(04)93639-3. PMID: 15235533. https://pubmed.ncbi.nlm.nih.gov/15235533/
6. Wikipedia contributors. Zinc compounds. Wikipedia, The Free Encyclopedia. April 21, 2024, 15:18 UTC. Available at: https://en.wikipedia.org/w/index.php?title=Zinc_compounds&oldid=1220054805.
7. https://health.ec.europa.eu/publications/water-soluble-zinc-salts-used-oral-hygiene-products-submission-ii_en
8. https://www.inchem.org/documents/pims/chemical/zincsalt.htm
9. https://www.dcceew.gov.au/environment/protection/npi/substances/fact-sheets/zinc-and-compounds
10. BOTT, W. Salts of Zinc. Nature 23, 78 (1880). https://doi.org/10.1038/023078c0
11. https://www.hsnstore.eu/blog/nutrition/minerals/zinc/salts-according-to-their-bioavailability/?srsltid=AfmBOooQqLziu_qWDuQfXhGK3590DdZxLndO1fYdk_O_QJW6gYj9fBmY
12. Alloteau F, Majérus O, Valbi V, et al. Efficacy of zinc salts to protect glass against atmospheric alteration. Part I: Effects of a spraying treatment. J Am Ceram Soc. 2021; 104: 2039–2051. https://doi.org/10.1111/jace.17590
13. https://www.cir-safety.org/sites/default/files/zincst122017TR.pdf
14. Almoudi MM, Hussein AS, Mohd Sarmin NI, Abu Hassan MI. Antibacterial effectiveness of different zinc salts on Streptococcus mutans and Streptococcus sobrinus: An in-vitro study. Saudi Dent J. 2023 Nov;35(7):883-890. doi: 10.1016/j.sdentj.2023.07.003. Epub 2023 Jul 6. PMID: 38025600; PMCID: PMC10658393. https://pubmed.ncbi.nlm.nih.gov/38025600/
15. https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/fs_G-87_1-Aug-92.pdf
16. Chen, L., Yu, X., Ding, H. et al. Comparing the Influence of High Doses of Different Zinc Salts on Oxidative Stress and Energy Depletion in IPEC-J2 Cells. Biol Trace Elem Res 196, 481–493 (2020). https://doi.org/10.1007/s12011-019-01948-4
17. Fehmida Fasim, Nuzhat Ahmed, Richard Parsons, Geoffrey M Gadd, Solubilization of zinc salts by a bacterium isolated from the air environment of a tannery, FEMS Microbiology Letters, Volume 213, Issue 1, July 2002, Pages 1–6, https://doi.org/10.1111/j.1574-6968.2002.tb11277.x
18. Pourrahimi, A. M.; Liu, D.; Pallon, L. K. H.; Andersson, R. L.; Martínez Abad, A.; Lagarón, J.-M.; Hedenqvist, M. S.; Ström, V.; Gedde, U. W.; Olsson, R. T. . (2014). Water-based synthesis and cleaning methods for high purity ZnO nanoparticles – comparing acetate, chloride, sulphate and nitrate zinc salt precursors. RSC Advances, 4(67), 35568–.doi:10.1039/c4ra06651k. https://pubs.rsc.org/en/content/articlelanding/2014/ra/c4ra06651k
19. Journal of Physical and Chemical Reference Data 21, 941 (1992); https://doi.org/10.1063/1.555909
20. Sorger, K. and Petersen, H. and Stohrer, J., Removal of zinc salts from nonaqueous synthesis solutions comprising zinc alkoxides or zinc amides, US6921487B2, United States https://patents.google.com/patent/US6921487B2/en
21. S. Salts of Zinc. Nature 23, 78 (1880). https://doi.org/10.1038/023078b0.
22. https://www.webmd.com/drugs/2/drug-64358/white-petrolatum-zinc-salts-topical/details
23. Wikipedia contributors. Zinc sulfate. Wikipedia, The Free Encyclopedia. August 23, 2024, 07:05 UTC. Available at: https://en.wikipedia.org/w/index.php?title=Zinc_sulfate&oldid=1241807211
24. Du, H., Dong, Y., Li, Q.-J., Zhao, R., Qi, X., Kan, W.-H., Suo, L., Qie, L., Li, J. and Huang, Y. (2023), A New Zinc Salt Chemistry for Aqueous Zinc-Metal Batteries. Adv. Mater., 35: 2210055. https://doi.org/10.1002/adma.202210055
25. Shankar, S., Rhim, JW. Effect of Zn salts and hydrolyzing agents on the morphology and antibacterial activity of zinc oxide nanoparticles. Environ Chem Lett 17, 1105–1109 (2019). https://doi.org/10.1007/s10311-018-00835-z
26. https://proceedings-szmc.org.pk/public/old-doc/1998/The-antimicrobial-activity-of-different-zinc-salts.pdf
27. Akgun MC, Afal A, Unalan HE. Hydrothermal zinc oxide nanowire growth with different zinc salts. Journal of Materials Research. 2012;27(18):2401-2407. doi:10.1557/jmr.2012.258. https://www.cambridge.org/core/journals/journal-of-materials-research/article/abs/hydrothermal-zinc-oxide-nanowire-growth-with-different-zinc-salts/1930B04DEB63761338E76715CF60BAD0
Zinc salts are chemical compounds that consist of zinc ions combined with various anions (e.g., sulfate, chloride, acetate). They are commonly used in supplements, medications, and various industrial applications to provide the essential mineral zinc.
Zinc salts play a crucial role in numerous bodily functions, including immune support, wound healing, DNA synthesis, and cellular metabolism. They help in maintaining skin health, improving cognitive function, and supporting growth and development.
Zinc salts enhance immune function by supporting the production and activity of immune cells, including T cells and white blood cells. They also have antioxidant properties, reducing oxidative stress and inflammation, which are vital for a healthy immune response.
Common types of zinc salts include zinc sulfate, zinc gluconate, zinc acetate, zinc citrate, and zinc oxide. Each type varies in its bioavailability, absorption rate, and specific applications in health and industry.
Zinc gluconate and zinc citrate are often considered the most effective for supplements due to their high bioavailability and gentle effect on the stomach. Zinc sulfate is also widely used but may cause gastrointestinal discomfort in some individuals.
Side effects of zinc salts may include nausea, vomiting, diarrhea, and stomach cramps, particularly when taken in high doses. Long-term excessive use can lead to copper deficiency, altered immune function, and other health issues.
Yes, zinc salts are commonly prescribed to treat zinc deficiency. Zinc sulfate, in particular, is often used in clinical settings to correct zinc levels and alleviate symptoms associated with deficiency, such as impaired growth, hair loss, and weakened immunity.
The recommended daily intake of zinc varies by age, gender, and health status. For most adults, a daily intake of 8-11 mg of zinc is recommended. It’s important to follow dosage instructions on supplements or consult with a healthcare provider.
Foods naturally rich in zinc include oysters, red meat, poultry, beans, nuts, seeds, and whole grains. These foods contain zinc in forms that the body can easily absorb and utilize.
Zinc salts are generally safe for long-term use when taken at recommended doses. However, prolonged use of high doses can lead to side effects, including copper deficiency and impaired immune function. It’s advisable to consult with a healthcare provider for long-term use.
Zinc salts, such as zinc gluconate and zinc citrate, are highly bioavailable and well-absorbed, making them effective forms of zinc supplements. Compared to zinc oxide, which has lower bioavailability, zinc salts are often preferred for dietary supplementation.