-
Product Name:
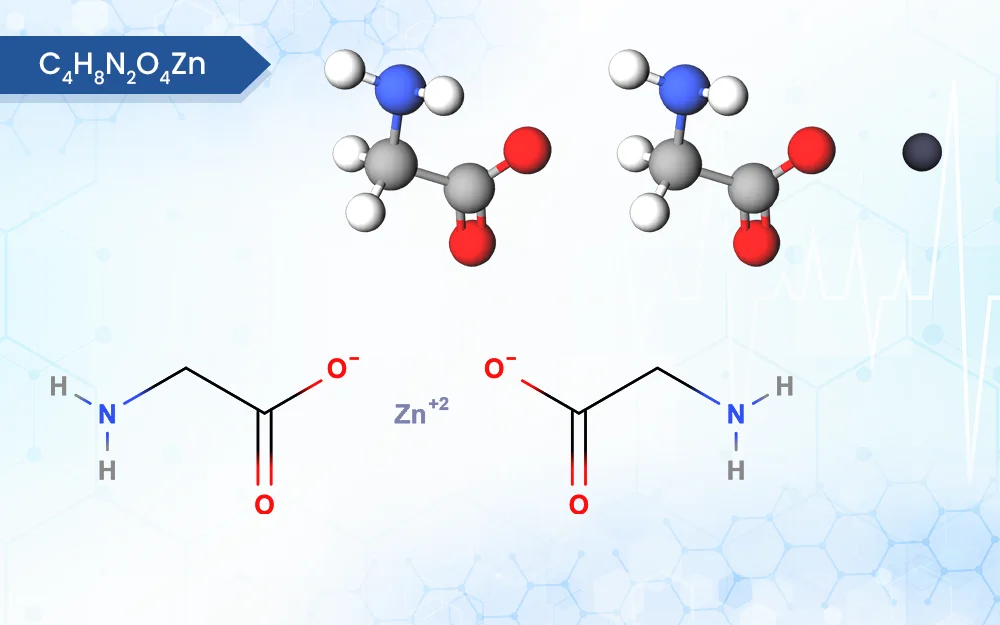
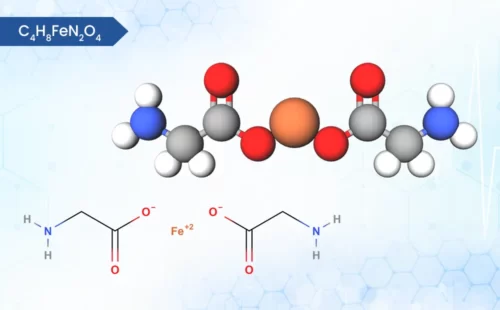
Zinc Bisglycinate
-
Molecular Formula:
C4H8N2O4Zn
-
Molecular Weight:
213.51 g/mol
-
CAS No.:
14281-83-5
-
HSN Code:
29224990
-
CID Code:
151910
-
Shelf Life:
3 years - 20°C powder
-
DrugBank ID
DB14493
-
ChemSpider ID
133891
-
UNII No.
681VJX72FE
- USP
- IUPAC Names
- Synonyms
- MSDS
USP of Zinc Bisglycinate
- This form of zinc is often used as a dietary supplement and is known for its high bioavailability.
- Zinc bisglycinate is absorbed in the small intestine. The presence of glycine in the compound aids in the transport of zinc through the intestinal wall, leading to higher absorption rates compared to other forms of zinc.
IUPAC Names of Zinc Bisglycinate
zinc;2-aminoacetate
Synonyms of Zinc Bisglycinate
- Zinc glycinate
- zinc glycine chelate
- zinc;2-aminoacetate
- Glycine Zinc Salt
- zinc bisglycinate
- Zinc(II) glycinate
- Zinc Chelazome
- Bis(glycinato-N,O)zinc
- Zinc(II) 2-aminoacetate
- zinc(2+) bis(2-aminoacetate)
- (T-4)-Bis(glycinato-N,O)zinc
- zinc 2-aminoacetate
- Zincglycinate
- UNII-681VJX72FE
- Zinc Bis-Glycinate
- zinc 2-azanylethanoate
- bis(glycinato)zinc
- Glycine Zinc Chelate
- Glycine Zinc Salt (2:1)
- Zinc Bis (Aminoacetate)
- Glycine Zinc Salt Monohydrate [for Protein Research]
- Zinc Amino Acid Chelate
- Zinc, bis(glycinato-kappaN,kappaO)-, (T-4)-
MSDS of Zinc Bisglycinate
Download MSDS PDF- Flammability of the Product: May be combustible at high temperature.
- Auto-Ignition Temperature: Not available.
- Flash Points: Not available.
- Flammable Limits: These products are carbon oxides (CO, CO2), nitrogen oxides (NO, NO2…). Some metallic oxides.
- Products of Combustion: Slightly flammable to flammable in presence of heat. Non-flammable in presence of shocks.
- Fire Hazards in Presence of Various Substances: Slightly explosive in presence of open flames and sparks. Non-explosive in presence of shocks.
- Fire Fighting Media and Instructions:
SMALL FIRE: Use DRY chemical powder.
LARGE FIRE: Use water spray, fog or foam. Do not use water jet. - Special Remarks on Fire Hazards: Fire is possible at elevated temperatures.
- Special Remarks on Explosion Hazards: Fine dust dispersed in air in sufficient concentrations, and in the presences of an ignition source is a potential dust explosion hazard.
- Engineering Controls: Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit.
- Personal Protection: Safety glasses. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves.
- Personal Protection in Case of a Large Spill: Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self-contained breathing apparatus should be used to avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product.
- Form: Free flowing powder, hygroscopic in nature.
- Color: White to off
- pH: 5.0 – 7.5
- Stability: The product is stable.
- Instability Temperature: Not available.
- Conditions of Instability: Excess heat, excess dust generation, incompatible materials.
- Incompatibility with various substances: Reactive with acids.
- Corrosivity: Not available.
- Special Remarks on reactivity: Incompatible with strong mineral acids, and strong organic acids.
- Special Remarks on Corrosivity: Not available.
- Polymerization: Will not occur
Description of Zinc Bisglycinate
Zinc Bisglycinate is a unique and well-tolerated form of zinc, a mineral essential for numerous bodily functions. Physically, it appears as a white, odourless powder with a slightly sweet taste. Unlike some other zinc supplements, it boasts low hygroscopicity, meaning it resists absorbing moisture from the air and clumping. This translates to a free-flowing, fine texture that’s easy to incorporate into capsules or tablets.
Chemically, Zinc Bisglycinate (Zn(C₄H₈N₂O₃) ₂) is a chelate formed when zinc ions bond with two glycine molecules, an amino acid known for its small size and sweetness. This chelation process enhances zinc’s bioavailability, essentially how well your body absorbs and utilizes the mineral. Compared to other zinc compounds, Zinc Bisglycinate is gentler on the digestive system, making it a popular choice for those sensitive to harsher forms of zinc.
As a leading API manufacturer, WBCIL prioritizes strict CGMP and ISO quality control measures throughout the production process. The prolonged experience from 1962 ensures consistent potency, purity, and safety in every dose of WBCIL’s Zinc Bisglycinate .
Application of Zinc Bisglycinate
Pharmaceutical Industry
- Nutritional supplements: Zinc gluconate can be formulated into tablets, capsules, or liquid supplements to treat zinc deficiency.
- Immune support products: It can be used in supplements designed to boost immune function, especially during cold and flu seasons.
- Cardiovascular health supplements: Zinc gluconate can be incorporated into heart health formulations.
Nutraceutical Industry
- Functional foods: Zinc gluconate can be added to fortified foods and beverages to enhance their nutritional value and provide immune support.
- Sports nutrition: It can be used in pre-workout or recovery supplements to support immune function and overall health in athletes.
Healthcare Industry
- Clinical nutrition: Zinc gluconate can be used in nutritional formulations for patients with compromised immune systems or those at risk of zinc deficiency.
- Geriatric care products: It can be incorporated into supplements designed specifically for elderly individuals to support their immune function and heart health.